Research led by Dr. Li-Fang (Jack) Chu at the University of Calgary Faculty of Veterinary Medicine is uncovering how species-specific developmental timing shapes early development, using pig stem-cells.

Li-Fang (Jack) Chu. Photo Credit: UCVM
Understanding how cells grow and change can unlock new therapies for regenerative medicine and Chu's team is showing encouraging results by creating retinal tissues similar to that found in human eyes.
Last year, the team's work, published in Stem Cell Reports, focused on porcine induced pluripotent stem cells (PiPSCs). These are pig cells reprogrammed into an embryonic-like state that can become any cell type in the body.
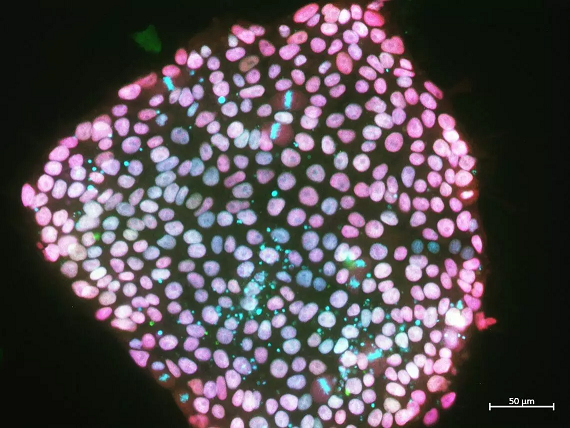
An undifferentiated pig pluripotent stem cell colony shows the presence of genes that help the cells stay in their stem cell state. Photo Credit: J. Vanessa Conrad, Chu Lab
In the first study, Chu's lab developed a transgene-free method to create PiPSCs safely, without permanently altering their DNA. These cells retained their natural developmental "clocks" in the lab, mirroring pigs' in vivo (in a living organism) timing. This allowed the team to model a process called the segmentation clock a rhythmic cycle that controls how embryos form body segments. In pigs, this cycle ticks every 3.7 hours, placing them between the fast-paced mouse clock (~2 hours) and the slower human clock (~5 hours).
"Pig stem cells preserve their species-specific pace, even outside the body," says Dr. Chu. "That's incredibly useful for studying how timing affects development."
From timing to tissues: building the pig retina
In a follow-up study published this month, in collaboration with Dr. David Gamm at the University of Wisconsin-Madison, the team used PiPSCsto grow retinal organoids 3D retina-like tissues containing light-sensitive photoreceptors.
However, the process wasn't straightforward. When the team applied standard human retinal differentiation protocols, the pig cells rarely formed retinal structures. The issue? The human protocol didn't match pigs' faster developmental timing or pace.
By shortening the timeline and fine-tuning molecular cues to reflect porcine development, the researchers developed a robust, reproducible method that generated hundreds of retinal organoids per experiment a major leap forward. The organoids contained organized layers of rods, cones, bipolar cells, and Müller glia (cells of the retina), with structural features essential for sensing light.
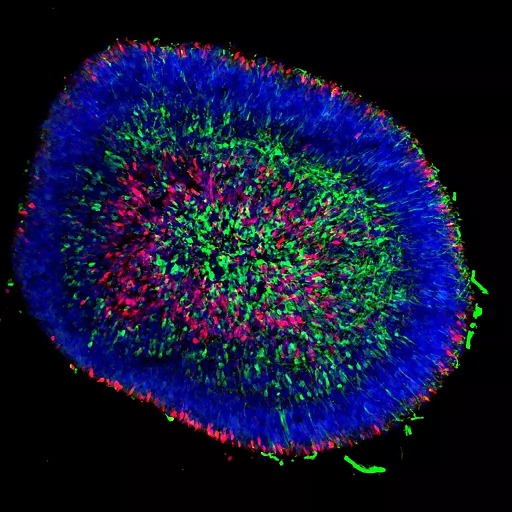
On day 40 of differentiation, pig stem cell-derived three-dimensional retinal organoids contained retinal ganglion cells (SNCG+ in green) and early photoreceptors (RCVRN+, in red). The cell nuclei are stained blue. Photo Credit: Kimberly L. Edwards, Gamm Lab (UW-Madison)
Importantly, these pig retinal tissues showed gene expression profiles remarkably similar to human retinal organoids, especially in photoreceptors, suggesting pigs can serve as a valuable model for testing cell-based therapies.
These findings have broad implications for regenerative medicine, veterinary science, and translational research. They highlight the value of pigs as a bridge species biologically closer to humans than rodents; for modeling development and testing therapies.
"This is about more than pigs," says Dr. Chu. "It's about finding the right models to understand how time shapes development and how we can use that to repair or replace tissues."
As the team continues cross-species comparisons, they also aim to uncover how mistimed development may underlie congenital conditions, and how stem cell technologies can help correct it.